EU-Projects
Perceptions of Uncertainty in Personalized Cancer Medicine (PCM) – A EU Survey Study
Background
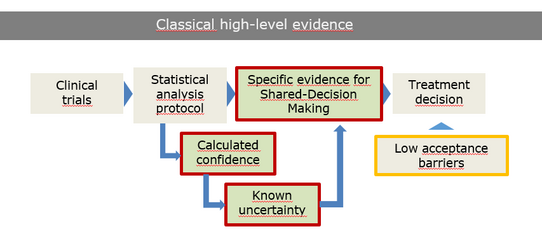
Precision oncology represents a fundamental paradigm shift: from guideline-based, population-level treatment to individualized, genomics-driven therapy tailored to the tumor biology. However, high-throughput, genome- or omics-based diagnostics introduce a new level of decisional uncertainty absent in traditional evidence hierarchies:
- weak analytical confidence in variant interpretation,
- low-level evidence for off-label applications,
- inconsistent data standardization across laboratories, and
- unclear real-world clinical benefit of personalized recommendations.
These uncertainties create acceptance barriers and challenge informed consent processes consequently affecting shared decision-making between clinicians and patients. Understanding how patients, clinicians, and molecular diagnosticians perceive and navigate these emerging uncertainties is critical for optimizing implementation of molecular tumor boards (MTB), ensuring appropriate informed consent management, and harmonizing precision medicine practice across European health systems.

For a deeper understanding of these potential barriers and creation of a scientific implementation background for PCM we will conduct a pan-European study on perception by various involved stakeholders. This will accompany the current activities within the two large cancer related EU-Joint Actions (EUnetCCC, JA PCM).
Objectives
Characterize and compare perceptions of uncertainty in PCM across three key stakeholder groups —patients, clinicians, and molecular diagnostic professionals — involved in MTB workflows at European university cancer centers. Our goal is to include at least 5.000 participants in 50 institutions in 10 European countries.
Methods
Prospective, multicenter, cross-sectional survey study. Structured and validated questionnaires assess uncertainty perceptions across stakeholder groups
- Patients: Advanced cancer patients (≥18 years old)
- Patient was discussed in a molecular tumor board at least once within the past 24 months OR received an MTB recommendation OR presents with advanced disease in palliative stage
- Disease Status: Metastatic or recurrent disease after exhaustion of at least one standard therapy line
- Clinicians: Clinically active physicians directly involved in the care of cancer patients. Where institutional access to MTBs exists, clinical experience with MTB case presentations or discussions is preferred.
- Molecular diagnosticians: Non-physician diagnostic partners involved in preparation or performance of molecular diagnostics without direct patient contact. This includes, for example, molecular biologists, molecular pathologists, bioinformaticians, laboratory scientists, and related roles contributing to genomic data generation, analysis, interpretation, or reporting within MTB workflows.
Contact & Study Participation
eu-projects@ccc-mv.de
